Time-resolved Hard X-ray Spectroscopy
(See LCLS Instruments for further details and Standard Configurations for hard X-ray scattering and spectroscopy: XPP, XCS, MFX)
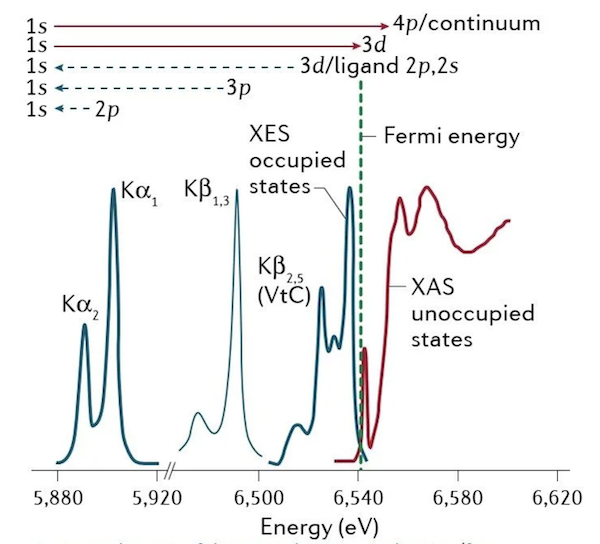
X-ray spectroscopy (illustrated in Fig. 1) is used to characterize electronic and geometric structures of elements in their chemical environment, exploiting the element-specificity of the core-level binding energies, and their further sensitivity to the local chemical environment (which imposes small energy shifts on the binding energies).
X-ray absorption spectroscopy (XAS) probes the electronic transitions from a core level to unoccupied valence states, whereas X-ray emission spectroscopy (XES) probes the fluorescence decay of occupied valence states to an empty core level (i.e. from an initially created core-excited state).
XAS and XES are complementary techniques providing information about local metal oxidation states, valence orbital populations and interactions, local metal spin states, ligand coordination, bond lengths and symmetry changes, and metal–ligand covalency.
K-edge XAS (hard X-rays) probes predominantly unoccupied molecular orbitals with p symmetry via 1s → 4p transitions. In the K-pre-edge region, weak quadrupolar 1s → 3d transitions can be observed, which gain intensity through mixing with orbitals with p symmetry.
L-edge XAS (soft X-rays) predominantly probes the orbitals with d symmetry via 2p → 3d transitions. These transitions relate to the low-energy region of an XAS spectrum (first few eV in the soft X-ray range and first few tens of an eV in the hard X-ray range), also referred to as the X-ray absorption near edge structure (XANES) region.
At higher energies (tens to hundreds of eV) is the extended X-ray absorption fine structure (EXAFS) region, which is dominated by scattering processes. In this case, the energy of the X-ray photons liberate photoelectrons that propagate from the absorber atom and are backscattered by neighboring atoms, producing interferences and the characteristic EXAFS oscillations that can be directly related to the atomic number, distance and coordination number of the atoms surrounding the metal absorber atom.
XES probes the transitions of an electron from an occupied orbital to an unoccupied or partially occupied core orbital. These transitions result from spontaneous fluorescence decays of core-excited states reached by X-ray absorption of the system. Non-resonant K-edge XES (Kα, Kβ1,3, Kβ′, Kβ2,5 and Kβ″), where the excitation energy is well above the core electron binding energy, provides information on the metal oxidation state, effective spin, metal bonding orbitals and nature of the ligand.
L lines in XES of 3d transition metals and K lines in XES of ligand atoms such as N or O provide sensitivity to oxidation states, symmetry, energies and interactions of occupied orbitals, and metal–ligand covalency. For a review of X-ray spectroscopy and applications to molecular systems see ref. [1].
U. Bergmann, J. Kern, R. W. Schoenlein, et al., "Using X-ray free-electron lasers for spectroscopy of molecular catalysts and metalloenzymes", Nature Reviews Physics 3, 264 (2021).
