Solvent Dynamics in Charge Transfer and Chemical Reactivity
LCLS studies in a model mixed-valence complex for photocatalysis illustrates the importance of understanding the role of solvent dynamics in mediating charge transfer and chemical reactivity.1 As shown in Fig. 1, the solvated cyanide-bridged mixed-valence bi-metallic charge-transfer complex, FeII-RuIII, is a model system for understanding how the complex couplings between electronic and atomic degrees of freedom in the solute and the surrounding solvent mediate the electron transfer (and back electron transfer) processes. Combined time-resolved X-ray scattering and spectroscopy studies of solvated FeII-RuIII at LCLS demonstrated that the dominant back electron transfer process is coupled to coherent translational motions of the first solvation shell. 1
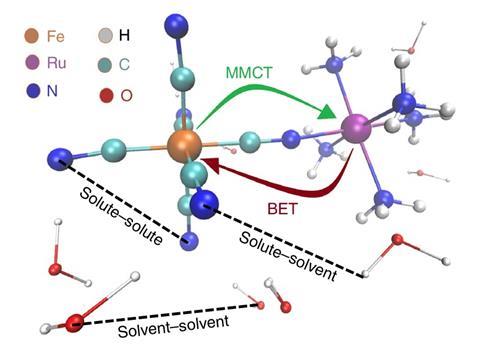
Figure 1. Mixed-valence aqueous FeII-RuIII molecule. Optical excitation induces metal-to-metal charge transfer (MMCT), followed by ultrafast back-election transfer (BET). Scattered X-rays reveal structural dynamics associated with solute-solute (intramolecular), solute-solvent, and solvent-solvent atom pair distances. 1
Comparison with non-equilibrium MD simulations demonstrate that the observed coherent translational motions arise from hydrogen bonding changes between the solute and nearby water molecules, in response to the initial photoexcitation. This result provides an atomistic view of coherent solvent reorganization (on the tenths of Å length scale and <100 fs time scale) mediating ultrafast intramolecular electron transfer.
E. Biasin, Z. W. Fox, A. Andersen, et al., "Direct observation of coherent femtosecond solvent reorganization coupled to intramolecular electron transfer", Nature Chemistry (2021).
